
Using phruta with a defined set of target genes
Source:vignettes/phruta_targetgenes.Rmd
phruta_targetgenes.RmdIn this vignette, we will be retrieving sequences from genbank using
phruta. We will need a list of taxa and the names of
particular gene regions that need to be sampled. If you have no idea on
what genes you should sample - phruta’ve got your back.
Just check the “Introduction to the `phruta` R package” and “To export
or not export `phruta` outputs” vignettes.
Now, given that we already have some target genes in mind, we can
avoid using gene.sampling.retrieve(). We won’t generate an
accession table using acc.table.retrieve() at the
beginning, mostly because the sampling that we’re using in
phruta this time. Therefore,
sq.retrieve.direct() is less flexible than
sq.retrieve.indirect(). As noted before,
sq.retrieve.direct() does its best to directly
(i.e. without input from the user) retrieve sequences for a target set
of taxa and set of gene regions. You are more likely to catch errors
using sq.retrieve.indirect(). However, mistakes will be
harder to spot and fix when using sq.retrieve.direct().
Let’s first start by loading phruta!
Let’s download gene sequences for the taxa in Felis,
Vulpes, and Phoca. Tjhis species-level sampling
corresponds with the one in other vignettes. We will be sampling only
two gene regions using sq.retrieve.direct(). Note that
we’re going to be using the list of species for the target genera from
the gbif taxonomic backbone (hence db = "gbif"). There are
more databases available for this (please check
taxize::downstream()).
sq.retrieve.direct(
clades = c("Felis", "Vulpes", "Phoca"),
species = "Manis_pentadactyla",
genes = c("ADORA3", "CYTB"),
db = "gbif"
)The code above will create a folder 0.Sequences in your
working directory. Note that sq.retrieve.direct() does not
return objects to the environment. The rest of this tutorial follow the
same structure as the “To export or not export `phruta` outputs”
vignette.
For instance, we can use the sq.curate() function to
remove highly divergent sequences. This function will also remove
observations from species that are not within our target taxonomic
groups.
sq.curate(filterTaxonomicCriteria = 'Felis|Vulpes|Phoca|Manis',
kingdom = 'animals',
folder = "0.Sequences",
removeOutliers = FALSE,
minSeqs = 2)Let’s review the current sampling…
| OriginalNames | AccN | Species | file | OldSpecies |
|---|---|---|---|---|
| AY011246.1 Felis catus adenosine A3 receptor (ADORA3) gene, partial cds | AY011246 | Felis_catus | ADORA3.fasta | Felis_catus |
| GU167614.1 Vulpes lagopus adenosine A3 receptor (ADORA3) gene, exon 2 and partial cds | GU167614 | Vulpes_lagopus | ADORA3.fasta | Vulpes_lagopus |
| GU167677.1 Phoca largha adenosine A3 receptor (ADORA3) gene, exon 2 and partial cds | GU167677 | Phoca_largha | ADORA3.fasta | Phoca_largha |
| GU930874.1 Phoca vitulina adenosine A3 receptor (ADORA3) gene, partial cds | GU930874 | Phoca_vitulina | ADORA3.fasta | Phoca_vitulina |
| AY011251.1 Manis pentadactyla adenosine A3 receptor (ADORA3) gene, partial cds | AY011251 | Manis_pentadactyla | ADORA3.fasta | Manis_pentadactyla |
| MH558234.1 Felis silvestris bieti isolate FBIPHap2 NADH dehydrogenase subunit 5 (ND5) and NADH dehydrogenase subunit 6 (ND6) genes, complete cds; tRNA-Glu gene, complete sequence; and cytochrome b (CytB) gene, partial cds; mitochondrial | MH558234 | Felis_silvestris | CYTB.fasta | Felis_silvestris |
| MW267829.1 Felis catus isolate VA79_2017 cytochrome b (cytb) gene, partial cds; mitochondrial | MW267829 | Felis_catus | CYTB.fasta | Felis_catus |
| MN370575.1 Felis chaus isolate Jungle Cat 5 cytochrome b (cytb) gene, partial cds; mitochondrial | MN370575 | Felis_chaus | CYTB.fasta | Felis_chaus |
| OM970983.1 Otocolobus manul isolate 6-1-11B cytochrome b (cytb) gene, partial cds; mitochondrial | OM970983 | Felis_manul | CYTB.fasta | Otocolobus_manul |
| MK606132.1 Felis margarita haplotype AH NADH dehydrogenase subunit 5 (ND5) and cytochrome b (cytb) genes, partial cds; and tRNA-Thr gene and D-loop, partial sequence; mitochondrial | MK606132 | Felis_margarita | CYTB.fasta | Felis_margarita |
| KU378587.1 Vulpes cana isolate B.F.Y3 cytochrome b (cytb) gene, partial cds; mitochondrial | KU378587 | Vulpes_cana | CYTB.fasta | Vulpes_cana |
| MT795179.1 Vulpes corsac isolate SH21 cytochrome b (CYTB) gene, complete cds; mitochondrial | MT795179 | Vulpes_corsac | CYTB.fasta | Vulpes_corsac |
| EU872065.1 Vulpes ferrilata haplotype 1 cytochrome b (cytb) gene, partial cds; mitochondrial | EU872065 | Vulpes_ferrilata | CYTB.fasta | Vulpes_ferrilata |
| KX093945.1 Vulpes lagopus haplotype 5 cytochrome b (cytb) gene, partial cds; mitochondrial | KX093945 | Vulpes_lagopus | CYTB.fasta | Vulpes_lagopus |
| AF028157.1 Vulpes macrotis cytochrome b (cytb) gene, mitochondrial gene encoding mitochondrial protein, partial cds | AF028157 | Vulpes_macrotis | CYTB.fasta | Vulpes_macrotis |
| KJ597964.1 Vulpes pallida haplotype PMa2 cytochrome b (cytb) gene, partial cds; mitochondrial | KJ597964 | Vulpes_pallida | CYTB.fasta | Vulpes_pallida |
| KU378373.1 Vulpes rueppellii isolate R.F.Y6 cytochrome b (cytb) gene, partial cds; mitochondrial | KU378373 | Vulpes_rueppellii | CYTB.fasta | Vulpes_rueppellii |
| MK244494.1 Vulpes vulpes isolate LD124 haplotype FOX cytochrome b (CYTB) gene, partial cds; mitochondrial | MK244494 | Vulpes_vulpes | CYTB.fasta | Vulpes_vulpes |
| MH854561.1 Vulpes zerda isolate X161349 cytochrome b (Cytb) gene, partial cds; mitochondrial | MH854561 | Vulpes_zerda | CYTB.fasta | Vulpes_zerda |
| LC466150.1 Phoca largha PLCBNo6 mitochondrial cytb gene for cytochrome b, partial cds | LC466150 | Phoca_largha | CYTB.fasta | Phoca_largha |
| AB510422.1 Phoca vitulina stejnegeri mitochondrial cytb gene for cytochrome b, complete cds, isolate: Pvs15 | AB510422 | Phoca_vitulina | CYTB.fasta | Phoca_vitulina |
| OP748355.1 Manis pentadactyla voucher MBSV033 cytochrome b (cytb) gene, partial cds; mitochondrial | OP748355 | Manis_pentadactyla | CYTB.fasta | Manis_pentadactyla |
Now, we’ll align the sequences that we just curated. For this, we use
sq.aln() with under default parameters.
sq.aln(folder = '1.CuratedSequences', FilePatterns = "renamed")The resulting multiple sequence alignments will be saved to
sqs.aln() object, a list. For each of the gene regions, we
will have access to the original alignment (Aln.Original),
the masked one (Aln.Masked), and information on the masking
process. Let’s first check the raw alignments…
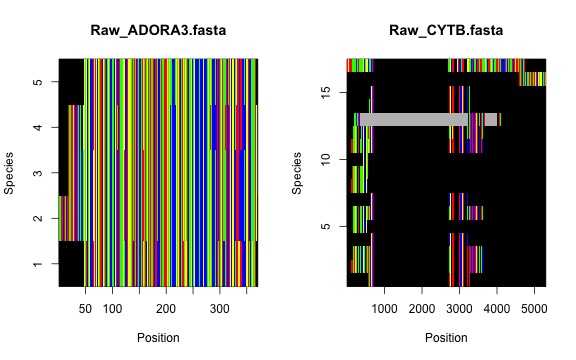 Now, the masked alignments…
Now, the masked alignments…
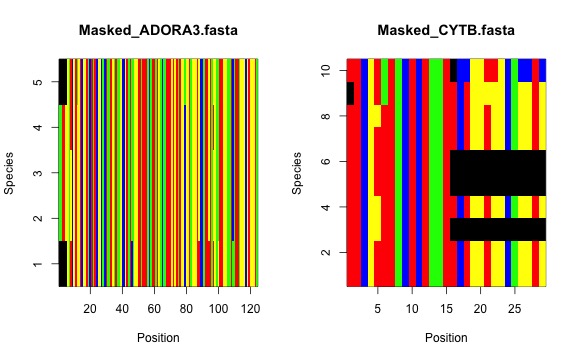
And we’re done for now!! You can compare the sampling between the
main three tutorials using sq.retrieve.direct() and
sq.retrieve.indirect(). In total, this vignette took 4
minutes to render in my local machine.