
Cleaning GBIF data for the use in biogeography
Source:vignettes/Cleaning_GBIF_data_with_CoordinateCleaner.Rmd
Cleaning_GBIF_data_with_CoordinateCleaner.RmdBackground
Big data aggregators such as the Global Biodiversity Information Facility (GBIF, www.gbif.org) have vastly increased the public availability of species occurrence records, with GBIF alone comprising more than 800 million records across all taxonomic groups. The data provided via these sources have revolutionized scientific biogeography and are highly valuable for research. However, some issues exist concerning data quality, mostly because these data are comprised from a variety of different collection methods (museum specimens, scientific surveys, citizen science, population counts for conservation purposes and genetic barcoding among others) and different sources (museums, herbaria, collections of individual researchers, citizen science, photo apps) and digitized and edited by various people and algorithms at different points in time and space.
In this tutorial we provide a pipeline on how to clean occurrence records retrieved from GBIF (or any other database) using CoordinateCleaner and meta data. The tutorial includes major steps we consider necessary, but by no means is complete and we explicitly encourage you to explore your data further before use. For the tutorial we will use a data set of occurrence records of a single species (lion, Panthera leo) downloaded from GBIF. On this example we can gauge the quality of cleaning steps, because we already have a good idea where we expect lions to occur. Of course, usually for multi-species data sets we do not have this kind of information, and that is the whole point of the automated cleaning. You can easily follow the tutorial using your own data instead. For the tutorial we will assume a global macroecological analysis with a resolution of about 100km as downstream analyses. Remember to adjust test sensitivity, if your analyses have a coarser or finer resolution.
With this tutorial you will be able to:
- Visualize the data and identify potential problems 2. Use CoordinateCleaner to automatically flag problematic records 3. Use GBIF provided meta-data to improve coordinate quality, tailored to your downstream analyses 4. Use automated cleaning algorithms of CoordinateCleaner to identify problematic contributing datasets
Identifying erroneous coordinates with CoordinateCleaner
The clean_coordinates function is a wrapper function
around all record-level tests of CoordinateCleaner. The idea
behind these tests is to use geographic gazetteers to identify records
that are most likely erroneous (or very imprecise). We based the choice
of tests on common problems observed in biological collection databases
(Maldonado et al., 2015), including
assignment to country centroids, sea coordinate and outliers among
others. You can get an overview over the individual tests using
?clean_coordinates or via the package
vignettes. This tutorial assumes occurrence data in the format as
downloaded from GBIF, for other formats you might need to adapt the
column names. You might need to install some of the required packages
for the tutorial using install.packages.
Install CoordinateCleaner
You can install the latest stable version of CoordinateCleaner from
CRAN using install.packages("CoordinateCleaner").
Alternatively you can install the latest development version from GitHub
using the devtools package. We recommend the latter, to stay up-to-date.
Also, make sure to have the latest R version installed.
install.packages("devtools")
library(devtools)
install_github("ropensci/CoordinateCleaner")Set up libraries and data
You might need to confirm to install the rnaturalearth package when
loading CoordinateCleaner
library(countrycode)
library(CoordinateCleaner)
library(dplyr)
library(ggplot2)
library(rgbif)
library(sf)
#obtain data from GBIF via rgbif
dat <- occ_search(scientificName = "Panthera leo",
limit = 5000,
hasCoordinate = TRUE)
dat <- dat$data
# names(dat) # a lot of columns
# select columns of interest
dat <- dat %>%
dplyr::select(species, decimalLongitude,
decimalLatitude, countryCode, individualCount,
gbifID, family, taxonRank, coordinateUncertaintyInMeters,
year, basisOfRecord, institutionCode, datasetName)
# remove records without coordinates
dat <- dat %>%
filter(!is.na(decimalLongitude)) %>%
filter(!is.na(decimalLatitude))Visualize the data on a map
#plot data to get an overview
wm <- borders("world", colour = "gray50", fill = "gray50")
ggplot() +
coord_fixed() +
wm +
geom_point(data = dat,
aes(x = decimalLongitude, y = decimalLatitude),
colour = "darkred",
size = 0.5) +
theme_bw()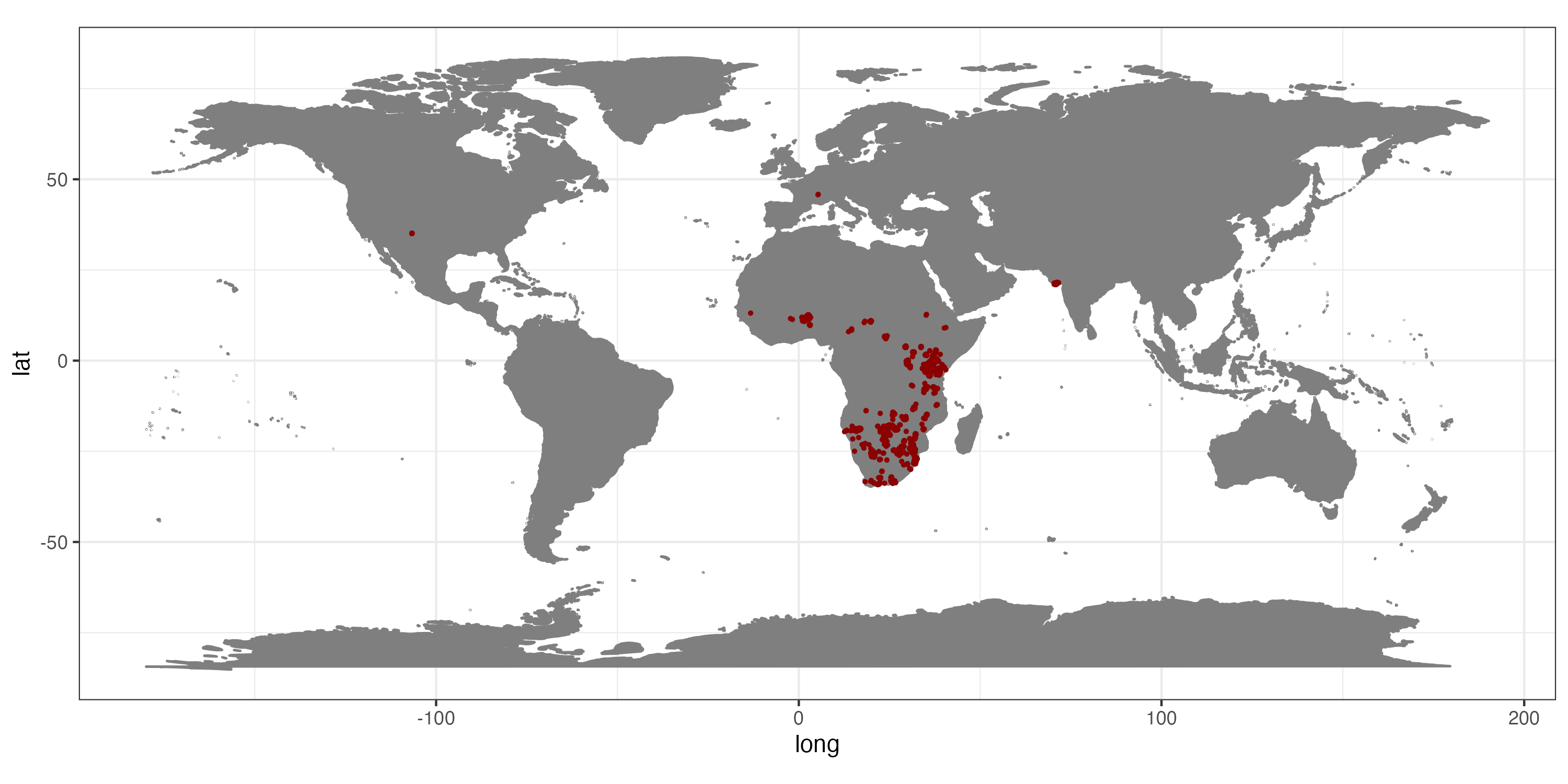
Occurrence records for Panthera leo obtained from GBIF.
This map clearly indicates, that we need to prepare the data further, if we want them to represent the current day (or historic) distribution of lions.
Use CoordinateCleaner to automatically flag problematic records
Option A) Using the clean_coordinates wrapper
function
As a first step we will run the automatic cleaning algorithm of
CoordinateCleaner. The clean_coordinates function is a
wrapper around a large set of automated cleaning steps to flag errors
that are common to biological collections, including: sea coordinates,
zero coordinates, coordinate - country mismatches, coordinates assigned
to country and province centroids, coordinates within city areas,
outlier coordinates and coordinates assigned to biodiversity
institutions. You can switch on each test individually using logical
flags, modify the sensitivity of most individual tests using the “.rad”
arguments, and provide custom gazetteers using the “.ref” arguments. See
?clean_coordinates for help. To use the country -
coordinate mismatch test we need to convert the country from ISO2 to
ISO3 format.
#convert country code from ISO2c to ISO3c
dat$countryCode <- countrycode(dat$countryCode,
origin = 'iso2c',
destination = 'iso3c')
#flag problems
dat <- data.frame(dat)
flags <- clean_coordinates(x = dat,
lon = "decimalLongitude",
lat = "decimalLatitude",
countries = "countryCode",
species = "species",
tests = c("capitals", "centroids",
"equal", "zeros", "countries")) # most test are on by default
## Testing coordinate validity
## Flagged 0 records.
## Testing equal lat/lon
## Flagged 0 records.
## Testing zero coordinates
## Flagged 0 records.
## Testing country capitals
## Flagged 36 records.
## Testing country centroids
## Flagged 1 records.
## Testing country identity
## Flagged 314 records.
## Flagged 350 of 5000 records, EQ = 0.07.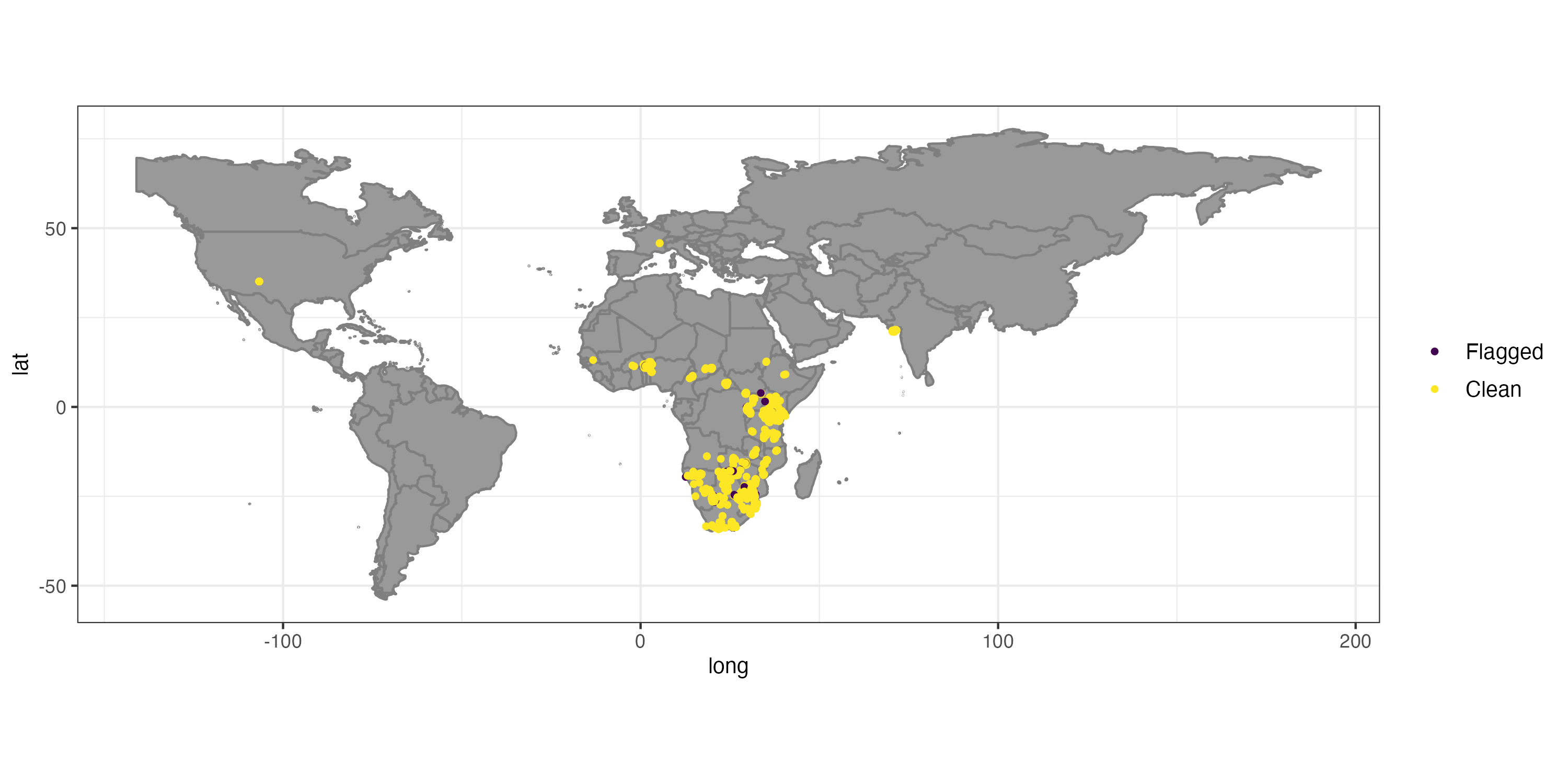
## .val .equ .zer .cap .cen .con .summary
## 0 0 0 36 1 314 350 The automatic test flagged 7% of the records. For the purpose of this tutorial we will exclude the flagged records, but in general it is recommendable to explore them further.
#Exclude problematic records
dat_cl <- dat[flags$.summary,]
#The flagged records
dat_fl <- dat[!flags$.summary,]Option B) Using the magrittr pipe (%>%)
Alternatively, you can run all tests implemented in
CoordinateCleaner with a individual function and connect them
using the magrittr pipe operator, which will directly result in a
data.frame comprising only cleaned records.
# To avoid specifying it in each function
names(dat)[2:3] <- c("decimalLongitude", "decimalLatitude")
clean <- dat %>%
cc_val() %>%
cc_equ() %>%
cc_cap() %>%
cc_cen() %>%
cc_coun(iso3 = "countryCode") %>%
cc_sea() %>%
cc_zero() %>%
cc_outl() %>%
cc_dupl()In this way, you can also add the individual test results as columns to your initial data.frame:
Temporal outliers
While the cc_outl function identifies geographic
outliers, record in GBIF migh also have doubtful temporal information,
i.e. for the time of collection, which can be problematic for example
for analyses of range dynamics. The cf_age function used
for fossil cleaning can also be used to check GBIF records for temporal
outliers.
flags <- cf_age(x = dat_cl,
lon = "decimalLongitude",
lat = "decimalLatitude",
taxon = "species",
min_age = "year",
max_age = "year",
value = "flagged")
# Testing temporal outliers on taxon level
# Flagged 0 records.
dat_cl <- dat_cl[flags, ]Improving data quality using GBIF meta-data
That helped a lot, but unfortunately some unwanted records remain, especially within Europe (Fig. ). This is mostly because we have used the occurrence records uncritically and ignored the meta-data. GBIF offers a whole lot of useful meta-data which we will use now to further refine quality of our dataset. First we’ll remove coordinates with very low precision and from unsuitable data sources. We will remove all records with a precision below 100 km as this represent the grain size of our downstream analysis, but we recommend you to chose it based on your downstream analyses. We also exclude fossils as we are interested in recent distributions; and records from unknown sources, as we deem them not reliable enough.
#Remove records with low coordinate precision
dat_cl %>%
mutate(Uncertainty = coordinateUncertaintyInMeters / 1000) %>%
ggplot(aes(x = Uncertainty)) +
geom_histogram() +
xlab("Coordinate uncertainty in meters") +
theme_bw()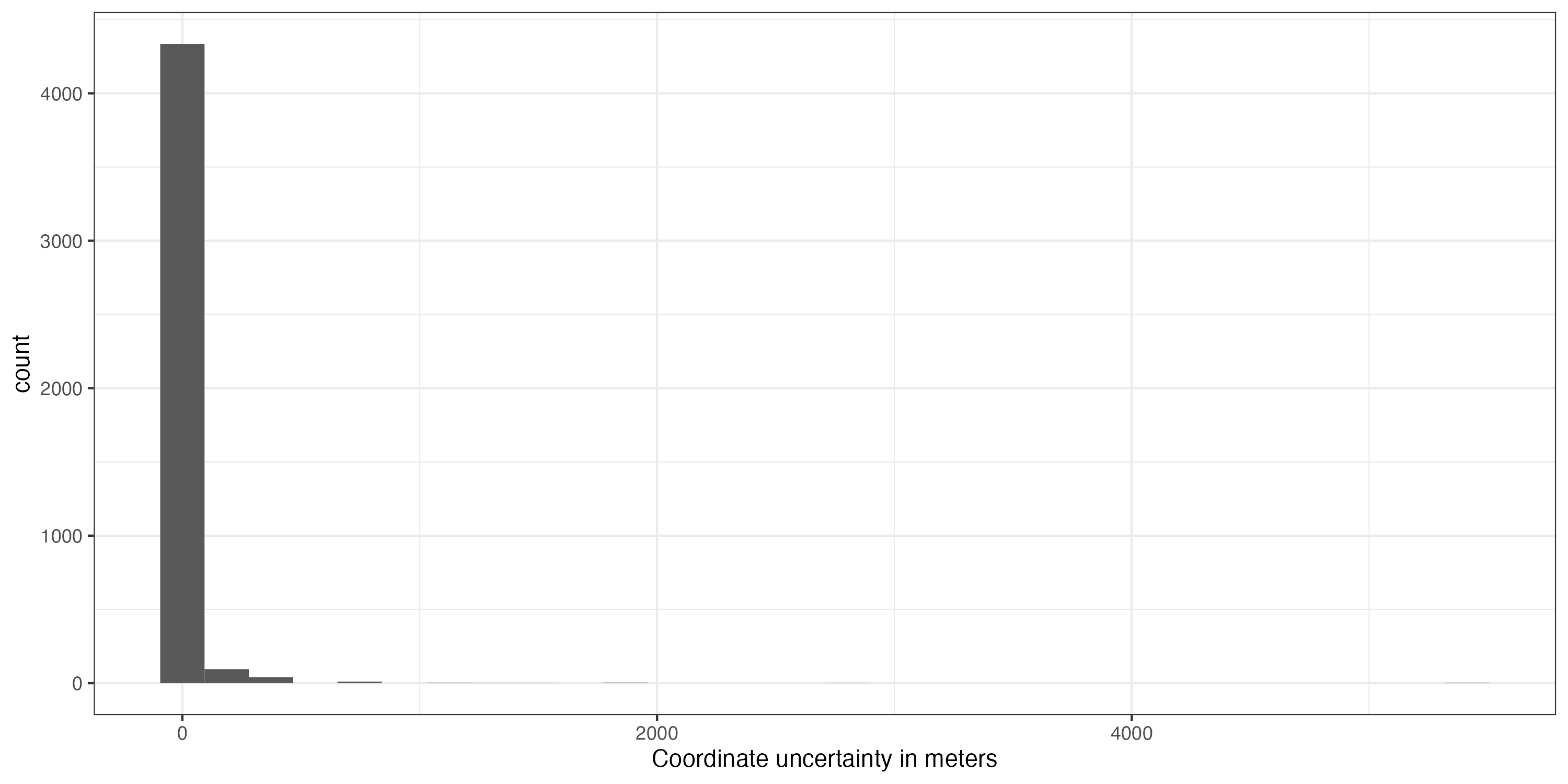
dat_cl <- dat_cl %>%
filter(coordinateUncertaintyInMeters / 1000 <= 100 | is.na(coordinateUncertaintyInMeters))
# Remove unsuitable data sources, especially fossils
# which are responsible for the majority of problems in this case
table(dat$basisOfRecord)
## HUMAN_OBSERVATION MATERIAL_SAMPLE PRESERVED_SPECIMEN
## 4979 2 19
dat_cl <- filter(dat_cl, basisOfRecord == "HUMAN_OBSERVATION" |
basisOfRecord == "OBSERVATION" |
basisOfRecord == "PRESERVED_SPECIMEN")In the next step we will remove records with suspicious individual counts. GBIF includes few records of absence (individual count = 0) and suspiciously high occurrence counts, which might indicate inappropriate data or data entry problems.
#Individual count
table(dat_cl$individualCount)##
## 1
## 84
dat_cl <- dat_cl %>%
filter(individualCount > 0 | is.na(individualCount)) %>%
filter(individualCount < 99 | is.na(individualCount)) # high counts are not a problemWe might also want to exclude very old records, as they are more likely to be unreliable. For instance, records from before the second world war are often very imprecise, especially if they were geo-referenced based on political entities. Additionally old records might be likely from areas where species went extinct (for example due to land-use change). Although this is not a problem in our dataset, we could still remove it with the following code.
#Age of records
table(dat_cl$year)## 2015 2016 2017 2018 2019 2020 2021 2022 2023
## 308 351 612 547 750 323 408 736 470 On top of the geographic cleaning, we also want to make sure to only include species level records and records from the right taxon. The latter is not a problem in this case, as we only have one species, but it can be helpful for large datasets. Taxonomic problems such as spelling mistakes in the names or synonyms can be a severe problem. We’ll not treat taxonomic cleaning here, but if you need to, check out the taxize R package or the taxonomic name resolution service (plants only).
table(dat_cl$family) #that looks good
##
## Felidae
## 4505
dat_cl <- dat_cl %>%
filter(family == 'Felidae')
table(dat_cl$taxonRank) # this is also good
##
## SPECIES SUBSPECIES
## 520 3985 We excluded almost 10% of the initial data points with the data cleaning, and the general picture has improved considerably. We confined the records mostly to what can be considered current day distribution of the species of interest (Fig. ). We have, however, also lost quite a number of records. In general, there is no “one-size-fits-it-all” for data quality of geographic species occurrence records. Of course highest coordinate precision is desirable, but what is acceptable will strongly depend on the downstream analyses. For species distribution modelling, usually high precision is necessary e.g. 1-10 km, but for other analyses such as biogeographic reconstructions using tectonic plates, a record might be considered good enough quality, as long as it is on the right continent. As another example for conservation purposes it might be sufficient to know that a species is present within a certain country.
Improving data quality using external information
Figure shows the success of automated cleaning. However, records within Europe and North America remain. A short inspection of the data suggests that these are a dubious human observation and five specimens, potentially assigned to their specimen location, or fossils with misclassified meta-data. One option to automatically flag these records is to rerun the outlier test on the cleaned data. However, this would most likely also flag the isolated Indian population (which is a true presence) as problematic.
Flag records based on fixed longitude and latitude
The first option alternative is to exclude records outside a certain study extent. In our example this is the easiest solution because we know that lions do not occur in high latitudes any more.
#exclude based on study area
dat_fin <- filter(dat_cl, decimalLatitude < 40)Flag records based on species natural ranges
In cases where simple latitudinal or longitudinal borders are not
useful, an alternative is to use species ranges from external source as
reference and flag all records falling outside these ranges. For
amphibians, birds, mammals and reptiles the International Union for the
conservation of nature (IUCN) provides detailed shape files of species’
natural distribution ranges. These can be downloaded for free at https://www.iucnredlist.org/resources/spatial-data-download.
CoordinateCleaner implements a straight forward way to use
these, or any other, ranges to flag records in the cc_iucn
function. Since downloading the IUCN shapes requires log-in we will
approximate lion’s natural range from scratch for our example. For
plants check out the botanical countries of The World Checklist of
Selected Plant Families facilitated by the Royal Botanic Gardens,
Kew.
#create simple natural range for lions
coords_range <- cbind(cbind(c(-23, -7, 31, 71, 83, 42, 41, 24, -23), c(14, 37, 32, 27, 18, 0, -16, -38, 14)))
wgs84 <- "+proj=longlat +datum=WGS84 +no_defs +ellps=WGS84 +towgs84=0,0,0"
nat_range <- terra::vect(coords_range, "polygons",
crs = wgs84)
nat_range$species <- "Panthera leo"
# Visualize range
plo <- sf::st_as_sf(nat_range)
## Regions defined for each Polygons
ggplot() +
borders("world", colour = "gray50", fill = "gray50") +
geom_sf(data = plo, aes(fill = species), alpha = 0.5) +
theme_bw() +
theme(legend.position = "none",
axis.title = element_blank())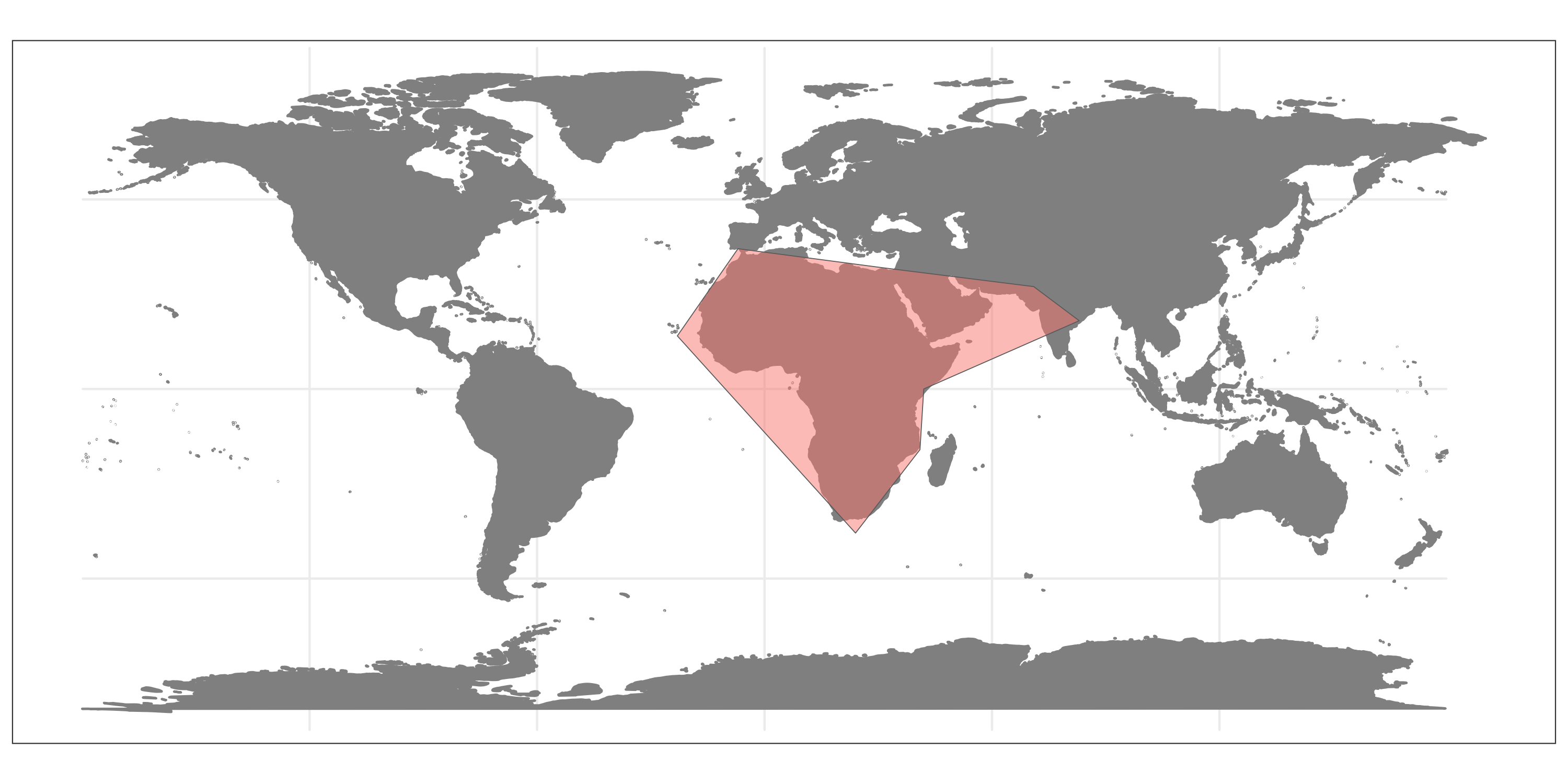
# run cc_iucn()
range_flags <- cc_iucn(x = dat_cl,
range = nat_range,
lon = "decimalLongitude",
lat = "decimalLatitude",
value = "flagged")
## Testing natural ranges
## Flagged 141 records.
## Warning message:
## In cc_iucn(x = dat_cl, range = nat_range, lon = "decimalLongitude", :
## reprojecting reference to '+proj=longlat +datum=WGS84 +no_defs'
dat_fin <- dat_cl[range_flags, ]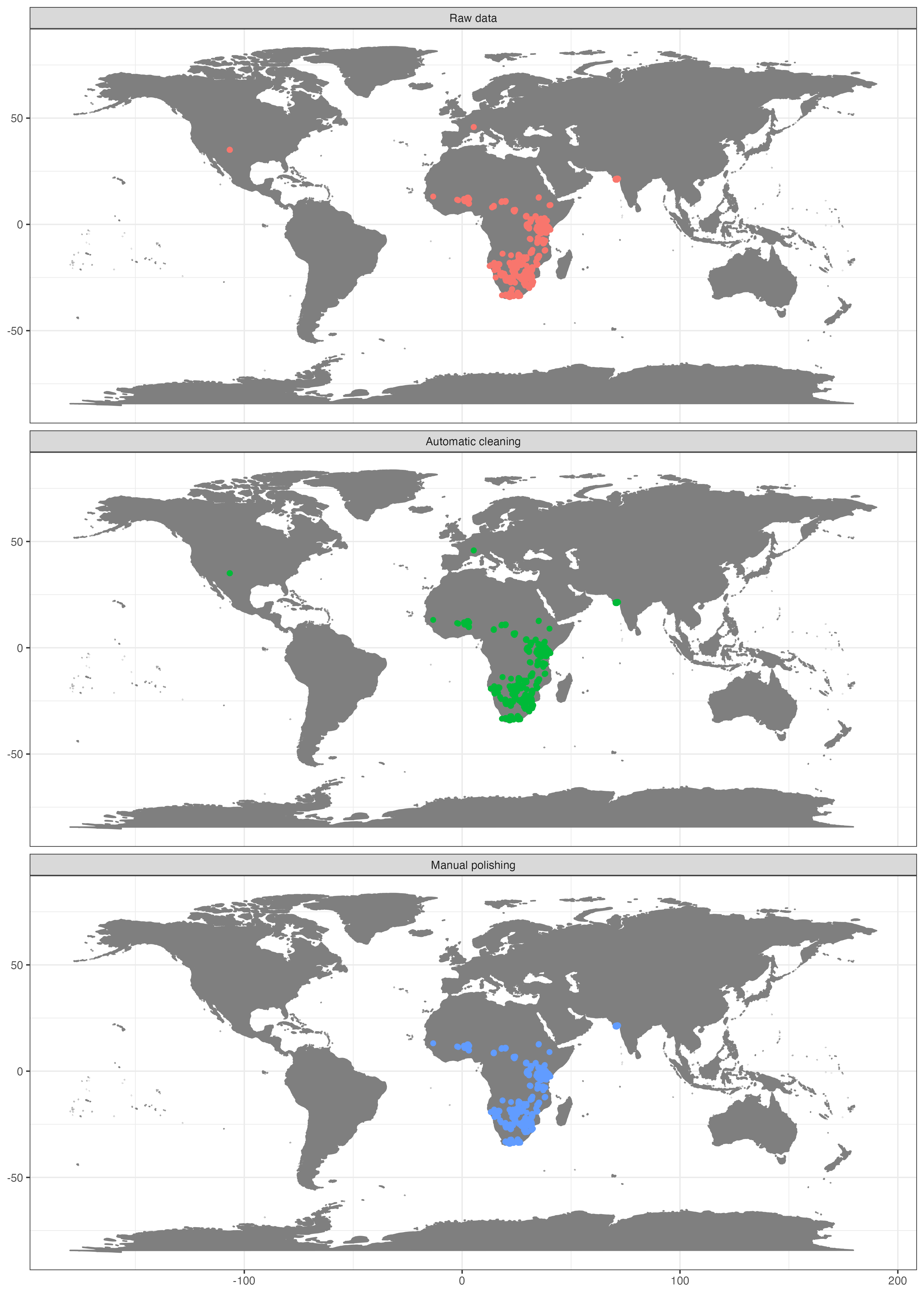
The dataset of occurrence of lions after different cleaning phases.
Identifying problematic data sets
Some types of potentially problematic coordinates can cause bias, but are not identifiable on record-level if the relevant meta-data are missing. This is especially the case if the erroneous records have been combined with precise GPS-based point occurrences into datasets of mixed precision. Two important cases are: (A) coordinate conversion errors based on the misinterpretation of the degree sign as decimal delimiter and (B) data derived from rasterized data collection designs (e.g. presence in a 50x50 km grid cell). CoordinateCleaner implements two algorithms to identify these problems on a dataset level.
Identify dataset with ddmm to dd.dd conversion error
We will first run the test for erroneous data conversion due to the
misinterpretation of the degree sign as decimal delimiter. We will use
the cd_ddmm function, alternatively, you can use the
clean_dataset wrapper. See supplementary material S1 for a
detailed description of the algorithm and implementation of the test.
You can control the output of the function via the value
argument.
out.ddmm <- cd_ddmm(dat_cl, lon = "decimalLongitude", lat = "decimalLatitude",
ds = "species", diagnostic = T, diff = 1,
value = "dataset")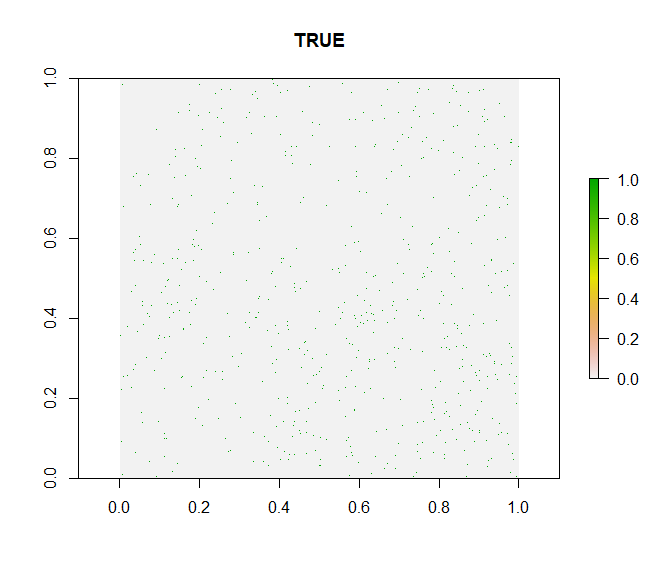
This looks good. The test indicates a slightly higher fraction of records with decimals below .60 than expected at random, but this is within the expected range and thus the test indicates no bias, which is confirmed by the diagnostic plot. In the case of a strong bias, the green points would be clustered in the bottom left quarter of the plot.
Test for rasterized sampling
As a second step we will use the cd_round function to
identify datasets with a significant proportion of coordinates that have
been collected in large scale lattice designs. These records might have
a low precision and might therefore be problematic for some analyses.
For instance presence derived from a 1 degree grid of a national atlas
might be to coarse for small scale species distribution models.
par(mfrow = c(2,2), mar = rep(2, 4))
out.round <- cd_round(dat_fin, lon = "decimalLongitude",
lat = "decimalLatitude",
ds = "species",
value = "dataset",
T1 = 7,
graphs = T)
## Testing for rasterized collection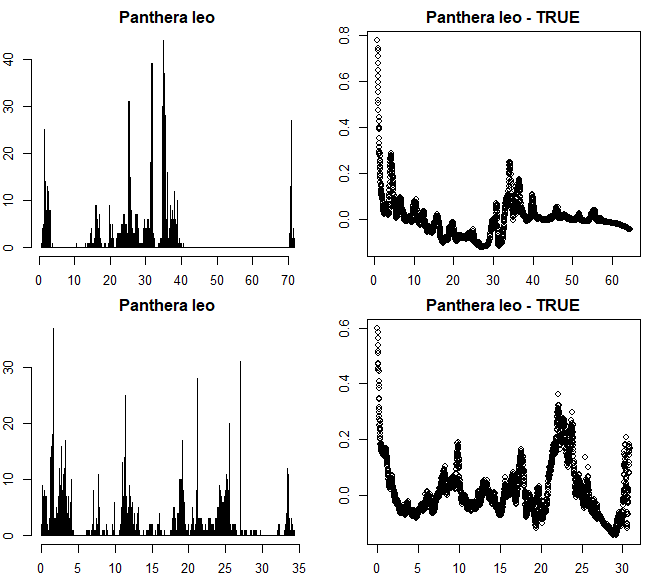
These results look good. The dataset does not show rasterized
collection schemes (see Supplementary material S1 for examples of biased
datasets). The test has detected and flagged some small scale and low
intensity periodicity in the longitude coordinates, however, the entire
dataset is only flagged if both longitude and latitude show a pattern
(as expected from rasterized sampling). You can modify the test
sensitivity using various arguments. See ?cd_round for more
information.
The lion dataset is relatively small and consistent, at least in the way that it only comprises on species. For larger scale analyses you might need to deal with larger datasets, composed from a larger variety of sources.